Prostate
Prostate Cancer Diagnosis and Tissue Ablation
For more than 2 decades, EDAP TMS has been committed to bringing accurate diagnosis tools and non-invasive alternatives to patients with prostate conditions with optimal efficacy and minimal side effects.
HIFU (High Intensity Focused Ultrasound), jointly developed in the early 1990s by Inserm (French Institute of Medical Research), HCL (Lyon University Hospitals) and EDAP TMS, has been used routinely to treat prostate cancer for more than 20 years throughout the world*. With more than 60,000 treatments performed on 3 generations of commercial devices: Ablatherm® Maxis (from 1993 to 2005), Ablatherm® Integrated Imaging (since 2005) and Focal One® (since 2013) HIFU has been used to treat prostate cancer* by creating a precise and irreversible coagulation necrosis of the targeted tissue while preserving the surrounding tissue.
MUS (Micro-Ultrasound) is a unique 29MHz ultrasound technology providing a resolution comparable to MRI and 300% improvement over traditional ultrasound. Developed by Canada-based company Exact Imaging™, the ExactVu™ system is the only device on the market capable of providing Micro-Ultrasound imaging to guide prostate biopsies and is exclusively distributed by EDAP TMS around the world.
ExactVu™ is a revolution in prostate cancer diagnosis. By bringing micro-ultrasound technology to visualize anatomical structures within the prostate, it allows targeting of suspicious areas, with or without MRI fusion, optimizing detection of significant cancer. Focal One® brings the answer to all requirements for ideal focal HIFU ablation: accurate and MR-fused imaging, non-invasive surgical approach, as well as precise and efficient therapeutic energy.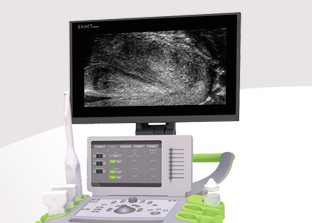
ExactVu™

Focal One®
* In the United States, Ablatherm® Fusion and Focal One® are indicated for the ablation of prostate tissue. HIFU can be subject to local regulations. For more information, please contact us. ExactVu™ micro-ultrasound system is available for sale in the European Union (CE Mark), the United States (FDA 510(k) clearance), and in Canada (Health Canada license). It can be subject to local regulations. For more information, please contact us.
HIFU can be subject to local regulations. For more information, please contact us.



