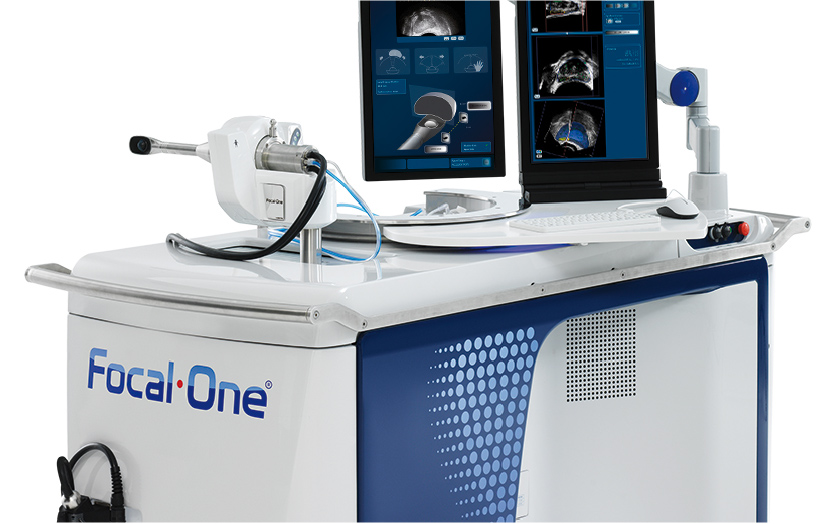
Less-invasive prostate cancer treatment receives FDA clearance
521tech - Posted July 3rd, 2018 - By Nicole Cobler
Prostate cancer patients around the country will soon have a less-invasive, radiation-free treatment option. French company EDAP TMS, which made Austin its U.S. headquarters two years ago, has received U.S. Food and Drug Administration clearance for “Focal One” — a treatment for prostate cancer using high-intensity focused ultrasound.