High Intensity Focused Ultrasound
Focal One®
Focal One® brings the answer to all requirements for ideal focal HIFU ablation: accurate and MR-fused imaging, non-invasive surgical approach, as well as precise and efficient therapeutic energy.
Visio-Track
Stereotactic Lithotripsy. "Freeline" ultrasound localization system. A user-friendly system to guarantee the best treatment efficacy. Available on the Sonolith® product range.
News
Mar 4, 2024
EDAP Announces FDA Breakthrough Device Designation for Focal One in the Treatment of Deep Infiltrating Rectal Endometriosis
Read more
Jul 26, 2023
EDAP TMS SA (Nasdaq: EDAP) (“the Company”), the global leader in robotic energy-based therapies, today announced that it has received reimbursement approval in Switzerland for the use of High-Intensity Focused Ultrasound (HIFU) in the treatment of prostate cancer. This reimbursement took effect on July 1, 2023.
Read more
Apr 21, 2023
Meeting to Showcase EDAP’s Focal One® Robotic High Intensity Focused Ultrasound (HIFU) Platform for the Management of Prostate Cancer
Read more
Apr 11, 2023
EDAP TMS SA, a global leader in robotic energy-based therapies, today announced that the Company will host a webcast that will feature a live procedure broadcast of its Focal One HIFU as a potential treatment for ablating cancer tissue in patients who are diagnosed with prostate cancer. The webcast is scheduled for Tuesday, April 18th at 12:00 p.m. PDT (3:00 p.m. EDT).
Read more
Mar 30, 2023
EDAP Announces Leadership Succession Plan to Develop Global Group Strategy
Read more
ESWL and stone laser
Stone Management
As a world leader in extracorporeal lithotripsy for more than 30 years, EDAP TMS offers a complete range of non-invasive and minimally invasive solutions covering the full scope of Urinary Tract Stone Indications.
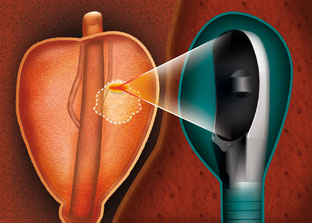
High intensity focused ultrasound
HIFU for Prostate Tissue Ablation
As an alternative to standard radical therapies to treat prostate conditions, EDAP TMS offers a non-invasive solution to ablate prostate tissue based on High Intensity Ultrasound (HIFU)…
Listen here!
Forbes Radio featured an interview with Dr. Miles, Prof. of Urology at Weill Cornell Medical College, an avid HIFU user.
Hemiablation prospective multicentric study of 111 patients now published in European Urology Journal. Link
HIFU Prostate website
was set up to provide patients with useful information relating to prostate conditions and particularly on the HIFU procedure.Link